Copaxone – EU Commission fines Teva €462.6 million over misuse of the patent system and disparagement.
https://ec.europa.eu/commission/presscorner/detail/en/ip_24_5581
Facts
Teva commercializes Copaxone for the treatment of multiple sclerosis, notably in the European Economic Area.
Copaxone has long been Teva’s largest blockbuster. The active agent of Copaxone is glatiramer acetate (or Copolymer-1), a random polymer composed of glutamic acid, lysine, alanine and tyrosine (4 amino acids found in myelin basic protein).
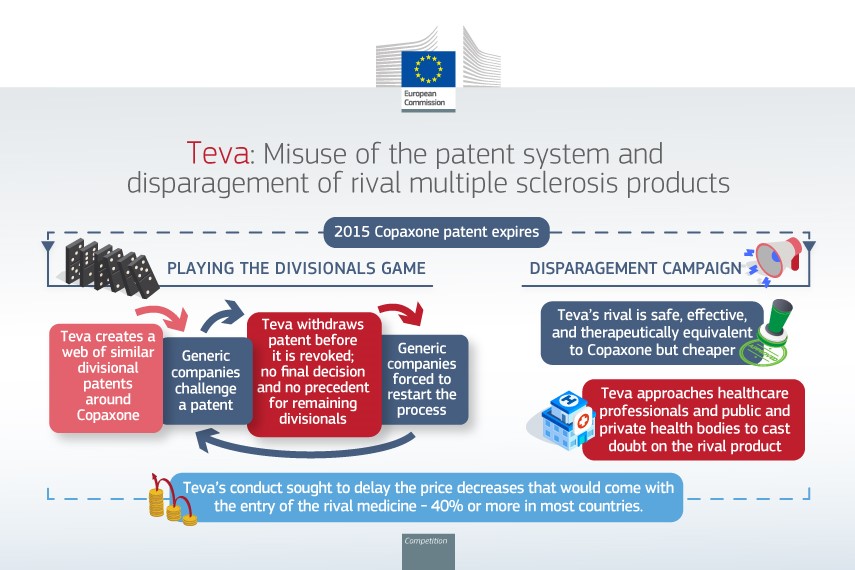
Teva was the exclusive licensee of the basic patent (EP0762888) protecting Copaxone that expired in 2015.
When the patent term came close to expiration, Teva filed numerous second patent applications and divisional applications derived therefrom, notably on the manufacture of the drug and specific dosage regimes, to create a patent network around Copaxone.
Competitors challenged these patents to clear the way to the market, especially before the EPO.
In parallel, Teva began to exert the patent rights – e.g. by interim injunctions – against generic companies looking to enter the market of glatiramer acetate.
When the patents appeared to be found invalid by the EPO, Teva withdrew them in order to avoid any revocation decision: this prevented the competitors from entering the market while avoiding adverse invalidity decisions being published by the EPO as well as by national courts. Teva’s conduct therefore forced the competitors to start the challenge process over and over again, thereby leading to third parties’ uncertainty.
According to the Commission, Teva also conducted a disparagement campaign to discredit a rival’s version of Copaxone.
Decision
Article 102 of the Treaty on the Functioning of the European Union (TFEU, as implemented by Regulation No 1/2003) prohibits the abuse of a dominant position within a market.
The EU Commission deemed that:
- Teva misused patent procedures: the strategy allowed Teva to artificially prolong legal uncertainty over its patents and, potentially, hinder the entry of competing/generic glatiramer acetate medicines ; and
- Teva implemented a systematic disparagement campaign against a rival’s version of Copaxone : it spread misleading information about its safety, efficacy and therapeutic equivalence with Copaxone, despite the relevant health authorities having approved the competing medicine and confirmed its safety, efficacy and therapeutic equivalence with Copaxone. The purpose was to slow down or block the entry of the rival product in several Member States.
This is the first time the Commission imposes a fine in relation to these two types of practices.
The case is available under number AT.40588 (https://competition-cases.ec.europa.eu/cases/AT.40588 ) but no public version of the decision is available for now due to business secrets/confidential information. The DG Competition is currently working on an editable version of the decision.
We will update this communication once the decision is made public.